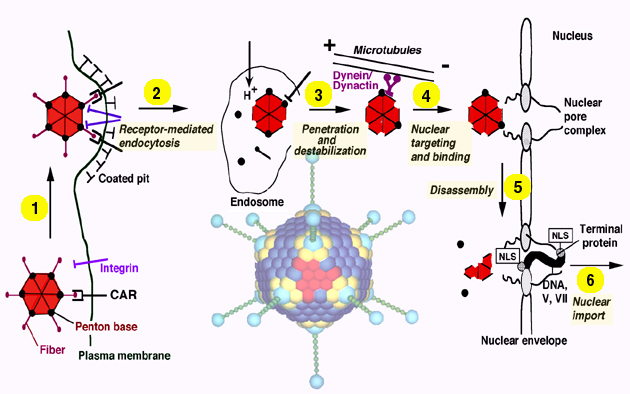

1 Adenovirus model
2 Endocytosis
3 Penetration
4 Microtubule-dependent nuclear targeting
5 Docking to nuclear pore complex and disassembly
6 Nuclear import of viral DNA
To initiate a new round of infection, extracellular virus attaches with high affinity to a target cell. A primary receptor of the immunoglobulin gene family, CAR (coxsackie virus, adenovirus receptor) has been identified as a specific determinant recognizing the distal fiber head domain of Ad2 and Ad5 (1; 2). An alternative receptor has also been identified as MHC class I (3). Entry into some cell types is facilitated by binding of penton base to a secondary receptor, alpha v-beta 3/5 integrins (4). Virus endocytosis then occurs through clathrin-coated vesicles (5-7) requiring the arginine-glycine-aspartate (RGD)-domain and possibly additional domains of penton base (4; 8).
Capsid dismantling is a stepwise process starting with fiber release at the time of virus endocytosis. Half of the incoming viruses loose their fibers within 10 min of internalization (9). After 30 min, more than 80% of the virions are fiber-less. The next proteins to be released are the capsid-stabilizing proteins IIIa and VIII. Protein IX, a cementing factor which keeps the hexons together is removed between 30 and 60 min after entry, long after the virus has reached the cytosol. Removal of protein IX, a capsid stabilizing component, coincides with, but is not sufficient for capsid disassembly. To liberate the DNA and dissociate the capsid reactivation of the capsid-resident cysteine protease p23 occurs. This process requires penton base-integrin interactions (and/or downstream events) and also reducing environment in endosomes or the cytosol, which facilitate p23-mediated protein VI degradation (10; 11). This step is thought to be essential to weaken the capsid and to prepare it for final dissociation and DNA import into the nucleus.
Besides protein VI degradation, import of viral DNA and associated protein VII into the nucleus also requires contact between the weakened capsid and cytosolic elements of the nuclear pore complex (NPC) (12). Specific inhibitors of O-linked glycoproteins of the NPC, such as the RL1 antibody or wheat germ agglutinin, microinjected into living cells block virus attachment to the nuclear membrane and inhibit capsid disassembly. It is possible that surface-exposed determinants of either hexon or penton base are involved in capsid attachment to the NPC. Whether capsid disassembly is directly triggered by an integral NPC component or indirectly, together with peripherally attached cytosolic components is not known. While docking and capsid dissociation occur independently of nuclear envelope calcium levels, nuclear DNA import is inhibited if calcium is depleted by ionophores or a calcium ATPase inhibitor, thapsigargin (12). These results are in agreement with earlier studies in cultured somatic cells and oocytes demonstrating a calcium-dependent function of the NPC (13; 14). The data suggest that the nuclear envelope can function as a regulatory barrier in nucleo-cytoplasmic trafficking of viral and cellular macromolecules. Whether the terminal protein, p55, which is covalently attached to the DNA and contains a transferable classical NLS (15), is somehow involved in threading the linear DNA through the NPC is not known.
(1). Bergelson, J. M., Cunningham, J. A., Droguett, G., Kurt-Jones, E. A., Krithivas, A., Hong, J. S., Horwitz, M. S., Crowell, R. L., and Finberg, R. W. (1997) Science 275, 1320-1323.
(2). Tomko, R. P., Xu, R., and Philipson, L. (1997) Proc. Natl. Acad. Sci. USA 94, 3352-3356.
(3). Hong, S. S., Karayan, L., Tournier, J., Curiel, D. T., and Boulanger, P. A. (1997) EMBO J. 16, 2294-2306.
(4). Wickham, T. J., Mathias, P., Cheresh, D. A., and Nemerow, G. R. (1993) Cell 73, 309-319.
(5). Svensson, U. (1985) J. Virol. 55, 442-449.
(6). Bai, M., Harfe, B., and Freimuth, P. (1993) J. Virol. 67, 5198-5205.
(7). Varga, M. J., Weibull, C., and Everitt, E. (1991) J. Virol. 65, 6061-6070.
(8). Stewart, P. L., Chiu, C. Y., Huang, S., Muir, T., Zhao, Y. M., Chait, B., Mathias, P., and Nemerow, G. R. (1997) EMBO Journal 16, 1189-1198.
(9). Greber, U. F., Willetts, M., Webster, P., and Helenius, A. (1993) Cell 75, 477-486.
(10). Cotten, M., and Weber, J. M. (1995) Virol. 213, 494-502.
(11). Greber, U. F., Webster, P., Weber, J., and Helenius, A. (1996) EMBO J. 15, 1766-1777.
(12). Greber, U. F., Suomalainen, M., Stidwill, R. P., Boucke, K., Ebersold, M., and Helenius, A. (1997) EMBO J. 16, 5998-6007.
(13). Greber, U. F., and Gerace, L. (1995) J. Cell Biol. 128, 5-14.
(14). Malviya, A. N., and Rogue, P. J. (1998) Cell 92, 17-23.
(15). Zhao, L.-J., and Padmanabhan, R. (1988) Cell 55, 1005-1015.